
FLUORIDATION FACTS
fluoridationfacts.com
B. Effect of Fluoride on the Permeability of the Erythrocyte Membranes for Na+, K+, Ca2+, F-, HPO42-, and Glucose
Exerting an influence on the permeability for the cations and anions at the cell membrane always has effects on the function of the effected cells. The effect can either support or hinder their cellular function.
In the case of nerve cells, for example, a depolarizing effect, which is the result of affecting the (Na+-K+) permeability of the membrane, can increase the excitability of the cell to a certain extent when the resting potential moves closer to the threshold potential without exceeding it. If the latter arises it leads to a constant depolarization and thereby un-excitability. The distribution of the (Na+-K+) ions is especially important because the polarization of the cell is the result of an unequal distribution of these ions between the intra- and extracellular spaces. We therefore tried, with the help of the radioactive isotopes Na-24 and K-42, to study the influence of the smallest F concentrations possible on the distribution of these ions across the cell membrane. The erythrocytes served as a model for the large number of other cells on which studies can only be carried out with a considerably larger experimental effort.
The separation of the (Na+-K+) ions at the membrane is an entropy reducing process. The necessary energy is taken from the splitting of ATP. SEN and POST(30) found that per split Mol of ATP, 3 Mol of Na+ are exported, and 2 Mol K+ are imported. The cell in turn extracts the ATP from glycolysis (Embden-Meyerhoff degradation) as well as the following respiratory chains. Erythrocytes are, however, because of missing sub-cellular particles, only capable of glycolysis. Every effect on the metabolism of ATP can therefore also have effects on active transport. Such an effect arises, for exampleDue to this variety of mechanisms one must study the effects of fluoride on as many parameters of the cellular medium as possible, whereby one must take great pains to match the remaining controlled variables as closely as possible to the natural conditions of the cell. We therefore studied the effect of fluoride on the variables listed above.
Description of the Isotopes Used
We carried out all subsequent studies with the help of radioactively labeled substances. The overwhelming portion of the tracers we used are commercially available (from Amersham-Buchler, Braunschweig). The isotopes 18F and 31Si are, because of their short life spans, not sold. We obtained the 18F in cooperation with the physics institute at the University of Hamburg (Prof. H. Neuert’s study group kindly took charge of 18F for us) while we produced the 31Si with the help of our institute's neutron generator. For now we could only study the production of 31Si from which we developed the basis for further studies (eg, resorption of hexafluorosilicates). The 18F was so far only twice available to us, because of which only a few orienting preliminary experiments could be carried out with it. In the following table we give an overview of the most important characteristics of the isotopes that were used.
Table 3. Radio-Isotopes Under Study
Key: Rad. = Radiation; Mev = Mega-Electron Volt; Prod. Proc. = Production Process.
| Isotope | Half-Life | Type of Rad. | Max Energy MeV | Prod. Proc. |
|---|---|---|---|---|
| 14C | 5730 years | b- | 0.156 | 14N(n,p) 14C |
| 18F | 1.83 hours | b+ | 0.65 | 16O(3He, p) 18F |
| g | 0.51 | |||
| 24Na | 15.5 hours | b- | 1.39 | 23Na(n,g) 24Na |
| g | 2.76 | |||
| 1.38 | ||||
| 31Si | 2.62 hours | b- | 1.48 | 31p(n,p) 31Si |
| g | 1.26 | |||
| 32P | 14.3 days | b- | 1.74 | 31P(n,g) 32p |
| 42K | 12.4 hours | b- | 3.58; 2.04 | 41K(n,g) 42K |
| g | 1.51; 0.32 | |||
| 45Ca | 165 days | b- | 0.25 | 44Ca(n,g) 45Ca |
Production of 31Si
Since we saw from the example of AChE that silicon compounds could be of biological importance, and since Si, like F, is probably one of the essential elements (daily release in the urine around 10mg), we undertook the task of developing a tracer method that would allow us to follow the path of Si in the body. The processes involved in resorption of SiF62- were of particular interest to us. Since radioactive isotopes of this element are not commercially sold, we developed a method to obtain carrier-free 31Si. Using our institute's neutron generator, which generates neutrons with an energy of 10-14 MeV at a maximum flow of 109 particles/sec and cm2, we exposed the purest red phosphorus to the neutron beam for 15 hours. An analysis of the g-spectrum of the specimen showed that only 28Al (half-life 2.31 min.) and 31Si had formed.
31P(n, p) 31Si s = 0.077b
31P(n, a) 28Al s = 0.15 b
The 28Al isotope did not, however, disrupt the experiment because of its relatively short half-life in comparison to 31Si. The following decay curve was derived by recording from a piece of the activated specimen in the liquid scintillation counter.
Figure 32 - Decay Curve of a Phosphorus Specimen After 15 Hours of Activation
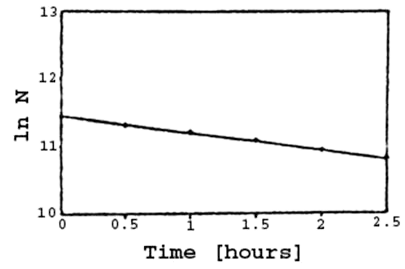
The mathematical expression for the curve in figure 32 forms the equation for the radioactive decay. In logarithmic form it reads:
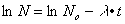
The following relationship develops between the decay constant, which is identical to the slope of the line here, and the half-life t1/2:
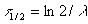
By inserting the value for the slope of the line from figure 32 one derives the half life as:
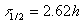
This value agrees exactly with the half life for 31Si (see table 3), which is an additional proof that only this radioactive isotope is present. Next we carried out a series of experiments to separate the red phosphorus from the carrier. We would like to briefly describe two of these experiments.
1.) We agitated a specimen of the activated phosphorus with 30% hydrofluoric acid. The Si, which due to the high degree of dispersion could be oxidized to SiO2 using the oxygen in the air, was supposed to react with the HF.
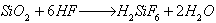
The hexafluorosilicic acid thereby went into solution. After a reaction time of one hour we separated the precipitates from the solution and determined the radioactivity in both the precipitate and the solution. Result: 15.5% of the total radioactivity was found in the solution.
2.) We agitated another specimen together with a 1% solution of MgSiF6 in water.
Result: After one hour 67% of the 31Si had gone into solution. So, a relatively fast exchange takes place on the phosphate carrier between the stable 30Si and the radioactive 31Si. This is, therefore, a very convenient method for separating the 31Si from the carrier, whereby a labeled SiF62- solution simultaneously forms and can be directly implemented in further experiments.
Unfortunately, the activities that can be derived in this way are too limited for many studies on biological systems (especially for studies on living animals). For this reason, in further studies we will have to rely on the production of this isotope from 30Si (over n, g reaction) in the reactor. The derived radioactivities (around 5 µCi) are, however, sufficient for measuring the permeability characteristics of fluorosilicate complexes of various biological membranes.
Previous Page: Westendorf Part 6 | Next Page: Westendorf Part 8